Media
HKU scientists reveal orally administrated bismuth drug together with N-acetyl cysteine as a potential broad-spectrum anti-coronavirus cocktail therapy
18 Jan 2022

Professor Hongzhe SUN (Norman & Cecilia Yip Professor in Bioinorganic Chemistry and Chair Professor of Chemistry) and his research team. From the right: Miss Suyu WANG (PhD student, Department of Chemistry); Dr Hongyan LI (Senior Research Assistant, Department of Chemistry); Professor Hongzhe SUN, Dr Runming WANG (Postdoctoral Fellow, Department of Chemistry); Dr Shuofeng YUAN (Assistant Professor, Department of Microbiology, HKUMed); Dr Jasper Fuk-Woo CHAN (Clinical Associate Professor, Department of Microbiology, HKUMed).
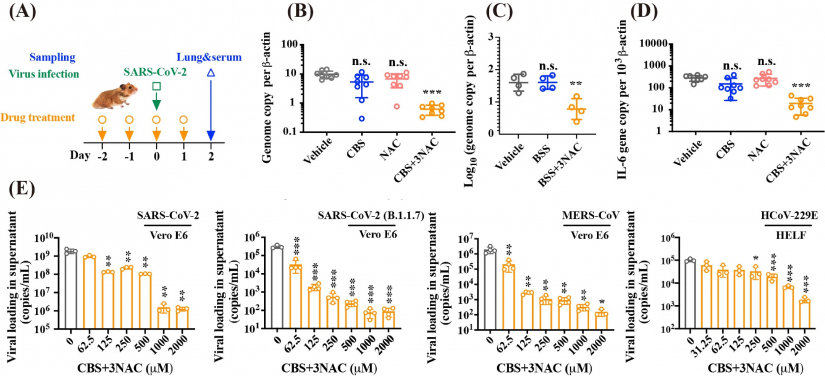
Combinatorial CBS and NAC exhibit broad-spectrum anti-CoVs potency both in vitro and in vivo. (A) Scheme depicting the therapeutic treatment via oral administration of vehicle, CBS (300 mg/kg) or BSS (300 mg/kg), NAC (370 mg/kg) and CBS (300 mg/kg)+3NAC (370 mg/kg) or BSS (300 mg/kg)+3NAC (405 mg/kg), given at Day -2, -1, 0 and 1 and the hamsters were challenged by virus at Day 0; Tissue samples were collected at two days after infection. (B) Viral yield in lung tissue of hamster receiving treatment of vehicle, CBS, NAC, and CBS+3NAC. (C) Viral yield in lung tissue of hamster receiving treatment of vehicle, BBS, and BSS+3NAC. (D) Cytokine IL-6 gene expression level. (E) CBS+3NAC suppressed replication of human-pathogenic coronaviruses in human cellular models in a dose-dependent manner, specifically for SARS-CoV-2 in Vero E6 cells; SARS-CoV-2 (B.1.1.7 variant) in Vero E6 cells; MERS-CoV in Vero E6 cell and HCoV-229E in HELF cell.
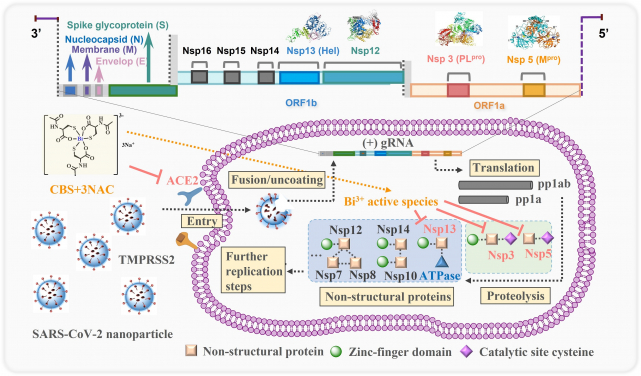
Proposed mechanism of action for orally administrated colloidal bismuth subcitrate together with N-acetyl cysteine as a broad-spectrum anti-coronavirus cocktail therapy. The pan-inhibitory activity of bismuth drugs against various CoVs may stem from their abilities to target multiple viral enzymes in the viral replication cycles. CBS as well as related metallodrugs could inactivate the viral cysteine protease through either targeting the key cysteine residue in the active site (PLpro and Mpro) or structural zinc-finger domain (PLpro and Hel) or even other zinc metalloproteins in human cells (ACE2) that are tightly associated with viral entry.
A research team led by Professor Hongzhe SUN, Norman & Cecilia Yip Professor in Bioinorganic Chemistry from the Department of Chemistry, Faculty of Science, the University of Hong Kong (HKU), in collaboration with Dr Shuofeng YUAN, Assistant Professor from the Department of Microbiology, Li Ka Shing Faculty of Medicine, discovered that orally administrated bismuth drug colloidal bismuth subcitrate (CBS) together with N-acetyl cysteine (NAC) could be a broad-spectrum anti-coronavirus cocktail therapy.
Oral administration of the cocktail suppresses the replication cycle of the virus, reduces viral loads in the lung and ameliorates virus-induced pneumonia in a hamster infection model. Not only could NAC stabilise bismuth-containing metallodrugs at stomach-like conditions but also enhance the uptake of bismuth drugs in tissues (e.g. lung) and antiviral potency through oral administration. Bismuth subsequently suppressed virus replication of a panel of clinically relevant coronaviruses, including Middle East respiratory syndrome-related coronavirus (MERS-CoV), Human coronavirus 229E (hCoV-229E) and Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its alpha variant (B.1.1.7) by inactivating multiple essential viral enzymes. The findings provided insights into the development of inorganic pharmaceutics and a new therapeutic approach for viral infections. The ground-breaking findings have been published in a leading chemical journal, Chemical Science and a related patent has been filed in the US.
Background
SARS-CoV-2 is the causative agent of the Coronavirus Disease 2019 (COVID-19) pandemic which leads to around five million confirmed cases, including 323,000 deaths globally. Although several vaccines have been approved for emergency use worldwide, increasing cases of people getting infected with COVID-19 are reported despite being fully vaccinated. The emergence of SARS-CoV-2 variants like Omicron and Delta variants associated with enhanced transmissibility and reduced sensitivity to vaccine-induced protection poses a continuous threat to global health. There is an urgent need for safe and effective therapeutic options for COVID-19 which remain scarce.
Remdesivir was the first drug approved by the US Food and Drug Administration (FDA) for the treatment of COVID-19. However, patients can only receive remdesivir treatment via intravenous route as in-patients as the oral formulation of this drug is still not available. For most COVID-19 patients with mild to moderate disease, an orally available anti-SARS-CoV-2 drug would help to facilitate out-patient treatment and reduce the burdens of healthcare facilities. Even though the US FDA issued emergency use authorisation for the oral tablet-form candidates from pharmaceutical giants Pifzer and Merck which reported significant reduction of the risk of hospitalisation or death, their antiviral efficacy, long-term safety and worldwide availability spark uncertainties. Therefore, it is of utmost urgency for renewed efforts to evaluate the existing repertoire of FDA-approved drugs on a wider scale and by a novel strategy.
Key findings
The research team previously screened a set of metallodrugs and related compounds and identified ranitidine bismuth citrate (RBC, commercial name: Pylorid), a drug in clinical use for the treatment of Helicobacter pylori infection, as a potent anti-SARS-CoV-2 agent both in vitro and in vivo. Pylorid exhibited low cytotoxicity and protected SARS-CoV-2-infected cells with a high selectivity index which demonstrated the high clinical potential of bismuth(III)-drugs or other metallodrugs for the treatment of SARS-CoV-2 infection. The related works were published in Nature Microbiology in 2020.
RBC, as well as other related bismuth drug(s), e.g., colloidal bismuth subcitrate (CBS) and bismuth salicylate (BSS), acts to precipitate in gastric juice and form a protective coating on the gastric wall, which leads to a hindered absorption in gastrointestinal tract. The findings revealed that NAC could prevent the hydrolysis of CBS in simulated gastric juice buffer (pH 1.2) and sodium bicarbonate buffer (pH 9.2), which form a highly stable and water-soluble Bi(III) thiolate complex, [Bi(NAC)3]. The combined use of NAC could significantly enhance the permeability of bismuth in parallel artificial membrane model, the human intestinal epithelial cancer cell line (Caco-2) model, and a modified ex vivo everted rat gut sac model. The thiolated bismuth could undergo fast thiol exchange with thiol groups in glycoproteins, which potentially increase both the lipophilicity and membrane permeability of bismuth, thus further enhancing the oral absorption of bismuth drugs. The in vivo pharmacokinetics data also consistently demonstrate that compared with the administration of CBS in the absence of NAC, the co-administration of CBS with NAC led to a remarkably improved bismuth uptake profile in both blood and lung tissues.
The studies demonstrated the oral efficacy of CBS+3NAC as well as BSS+3NAC on the suppression of SARS-CoV-2 replication in vivo as evidenced by the substantial reduction of viral loading in the lungs based on viral RNA genome copy number and the ameliorated virus-associated lung pathology after oral administration of CBS+3NAC. The therapeutic dosage of drugs induced only reversible nephrotoxicity and no systematic toxicities. More importantly, CBS+3NAC inhibits the replication of a broad range of epidemic and seasonal CoVs, including SARS-CoV-2 (B.1.1.7), MERS-CoV, and hCoV-229E. The pan-inhibitory activity of bismuth drugs against various CoVs may stem from their abilities to target multiple key viral cysteine enzymes in the viral replication cycles, including papain-like protease (PLpro), main protease (Mpro), helicase (Hel) and angiotensin-converting enzyme 2 (ACE2).
About the research team
The research was conducted by a team led by Professor Hongzhe SUN, Chair Professor of Chemistry, Department of Chemistry. Professor Hongzhe SUN and Dr Shuofeng YUAN, Assistant Professor from the Department of Microbiology, Li Ka Shing Faculty of Medicine are co-corresponding authors. Postdoctoral fellow Dr Runming WANG, Clinical Associate Professor Jasper Fuk-Woo CHAN and Miss Suyu WANG are co-first authors. Other HKU scientists contributing to the research included Dr Hongyan Li, Senior Research Assistant (Chemistry), Postdoctoral fellow Dr Jiajia Zhao (Pharmacy, CUHK), Miss Tiffany Ka-Yan Ip, PhD student (Chemistry), Professor Joan Zhong ZUO, Professor (Pharmacy, CUHK), Professor Kwok-Yung YUEN, Chair Professor (Department of Microbiology, HKUMed).
The work was supported by Innovation Research Commission, Research Grants Council, Health and Medical Research Fund of Hong Kong SAR, and The University of Hong Kong.
About Professor Hongzhe SUN and Dr Shuofeng Yuan
Professor Hongzhe SUN is a Norman & Cecilia Yip Professor in Bioinorganic Chemistry and Chair Professor of Chemistry at The University of Hong Kong. His research focuses on metalloproteins, the discovery of antimicrobial agents, and inorganic chemical biology.
More information about Professor Hongzhe SUN and his research group can be found from their group’s webpage: https://chemistry.hku.hk/staff/hzsun/labPage/index.html
Dr Shuofeng Yuan is an Assistant Professor at the Department of Microbiology and State Key Laboratory of Emerging Infectious Diseases, The University of Hong Kong.
More information about Dr Shuofeng Yuan, and his research group can be found from their group’s webpage: http://www.microbiology.hku.hk/02_HKU_Staff_Dr_SY_Yuan.html
About the research paper: https://doi.org/10.1039/D1SC04515F
Images download and captions: https://www.scifac.hku.hk/press
For media enquiries, please contact Ms Casey To, External Relations Officer of HKU Faculty of Science (tel: 39174948; email: caseyto@hku.hk / Ms. Cindy Chan, Assistant Director of Communications of HKU Faculty of Science (tel: 3917 5286; email: cindycst@hku.hk).
